Since we do not have a vaccine for COVID-19 yet, and vaccines normally take a year or more to develop, the concept of Herd Immunity has gotten a lot of bad press lately.
COVID-19 is a pandemic, meaning that we have failed to contain the spread of this virus from a particular region. Under the current circumstances, the pandemic can only end with something called Herd Immunity. Herd immunity depends on the proportion of the population that must be immune to a virus to stop its spread. So bad press or not, the need for Herd Immunity is, in fact, a reality.
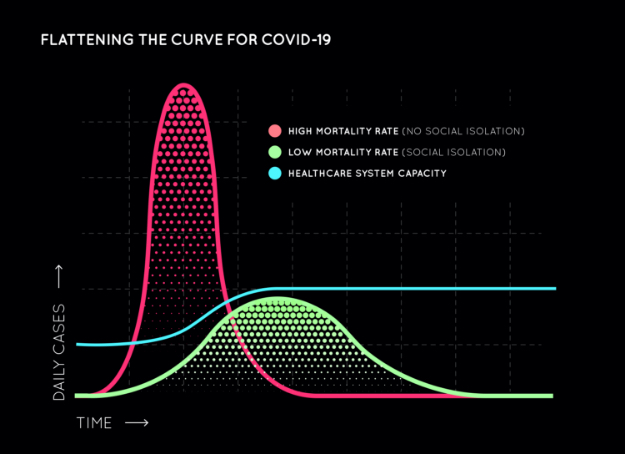
Countries like the UK, the Netherlands, and Sweden initially took liberal approaches, citing “Herd Immunity” was the only way to get through this, and opted against early social distancing. While theoretically true, there was one fundamental flaw in that initiative and they have since reversed that decision. Even if everyone must get sick to become immune without a vaccine, it’s not a good idea for everyone to get sick all at once. If hospital systems are overwhelmed, or personal protective equipment, medications, and ventilators are exhausted because everyone gets sick all at once, many, many more lives will be lost. Alternately, if the COVID-19-positive infection rate (red curve) is “flattened” with fewer infections per day (green curve), the spread of COVID-19 will be slowed to allow healthcare systems to cope, ie. the blue breakpoint line. Hence the importance of safety precautions and social distancing.
The question of the hour: what proportion of the population must become immune to achieve Herd Immunity? This percentage varies depending on a number of factors including modes of transmission, how contagious a disease is, and how long an infectious virus can live on surfaces. Upper respiratory viruses typically need at least 60-70% of the population to be immune to break the chain and control an outbreak. The more people immune, the better the control.
The goal of Herd Immunity is achieved in two ways:
1) Contraction of the disease, resulting in antibodies through natural immunity
2) Acquired immunity through vaccination. Without a vaccine on the horizon, this news sends people into a frenzy. With 300 million people in America, that would mean that 180 million people would need to contract the disease to control it, but there is more to it than that.
Upper respiratory viruses typically need at least 60-70% of the population to be immune to break the chain and control an outbreak.
The more people immune, the better the control.
Let’s consider some facts: As of now, we do not know how many people have already contracted COVID-19 with mild to minimal symptoms and are already immune to it. Once we have a better COVID-19 antibody test available, we will be able to determine who can go back to a more normal life first. Knowing this information will also give us a better perspective on demographics, risk factors and virulence, helping us to make wise research-driven decisions.
Thanks to advances in technology, important discoveries – including the architecture of COVID-19’s surface proteins that could be used to manufacture a vaccine – have been made faster than ever before. Because things like vaccines are usually proprietary, historically, research teams working independently take a long time to develop a vaccine. This pandemic has indeed brought together research teams, collaborating (rather than competing) world-wide on the development of both vaccines (Appendix A) and alternative therapeutic strategies.
Which brings us back to number one: natural immunity. While it may take many months (if we are lucky) to identify an effective vaccine in the laboratory, it will take nearly a year to further prove both efficacy and safety for human use. Meanwhile, there is a race to better understand the COVID-19 infection and how to prevent its severe complications. As more studies are done and treatment protocols are improved, we can get closer to figuring out how to decrease complications from infection before they arise. That’s pretty important because in the end, viruses can still mutate and we don’t know if COVID-19 can come back differently like the flu does each year. Current promising experimental treatments include antivirals (remdesivir and others), hydroxychloroquine with or without azithromycin, immune system modulators, IL-6 antibodies, antibody-rich plasma infusions donated by recovered patients, monoclonal antibodies that resemble antibodies from recovered patients, and ACE-2 competitive inhibitors, to name a few.
While Herd Immunity is a reality we must face to stop the spread of COVID-19, the way we manage reaching that tipping point safely is still evolving.
Appendix A: Information on companies currently working on vaccines from The Pharmaceutical Journal.1
• Organizations working on vaccine: Johnson & Johnson; Geovax Labs and BravoVax; University of Oxford and Advent Srl; Tonix Pharmaceuticals and Southern Research; Altimmune; Greffex; Vaxart; CanSino Biologics; Zydus Cadila; Institute Pasteur
• Estimated date of first human trials: June 2020
2. DNA vaccine:
• Organizations working on vaccine: Inovio Pharmaceuticals with Beijing Advaccine Biotechnology; Applied DNA Sciences, Takis Biotech and Evvivax; Zydus Cadila
• Estimated date of first human trials: April 2020
3. RNA vaccine:
• Organizations working on vaccine: CureVac; Moderna and US National Institute of Allergy and Infectious Diseases; Stermirna Therapeutics, Tongji University and Chinese Center for Disease Control and Prevention; Imperial College London
• Date of first human trials: March 2020
4. Live-attenuated vaccine:
• Organizations working on vaccine: Codagenix with Serum Institute of India
• Estimated date of first human trials: August 2020
5. Protein-based vaccine:
• Organizations working on vaccine: Novavax; Clover Biopharmaceuticals with GSK; Baylor College of Medicine, University of Texas Medical Branch, New York Blood Center and Fundan University, China; University of Saskatchewan, Canada; University of Queensland, Australia, and Dynavax; Vaxart; Generex; ExpreS2ion; Vaxil Bio; Sanofi Pasteur; iBio/CC-Pharming
• Estimated date of first human trials: By June 2020
- 1. Connoly D and Robinson J. The Pharmaceutical Journal. March 2020, Vol 304, No. 7935

